SMi Source lesson Drug R&D: Clinical Trial Design has the following microlearning topics
1. Drug Development Overview
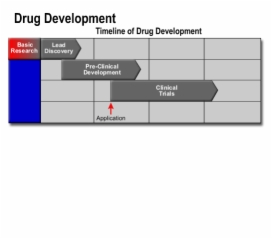

2. Clinical Trials in Drug Development


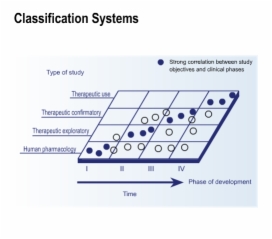
3. Study Objectives

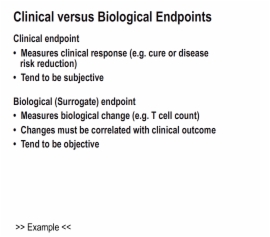
4. Endpoints

5. Minimizing Bias


6. Types of Studies
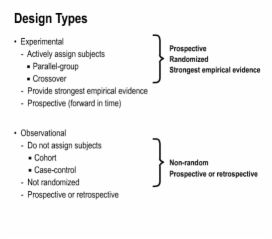

7. Application of Design
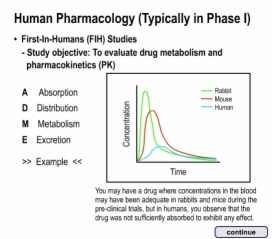

8. Conclusion
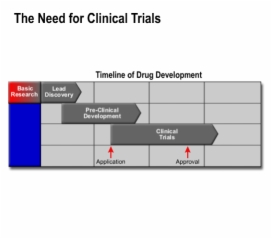

Lesson Drug R&D: Clinical Trial Design teaches these concepts
Clinical Trial Design, Drug Development Overview, Clinical Trial
Clinical Trial Design, Drug Development Overview, Drug Development
Lesson Drug R&D: Clinical Trial Design addresses these key points
Clinical Trial
- Research studies conducted with human subjects
- Mandated by regulatory agencies worldwide before any new drug reaches the marketplace
- Proper conduct of clinical trials is challenging, complex, time-consuming and expensive
- Bringing one new drug to market costs $1.3 billion and takes up to 15 years
- Approximately $626 million alone are needed just for the required clinical studies
Drug Development
- Computer modeling
- Automated testing
- Scientific meetings
- External collaborations
- Licensing opportunities