SMi Source lesson Biotech: Biosimilars has the following microlearning topics
1. Introduction to Biosimilars


2. Biologics and Protein Therapeutics
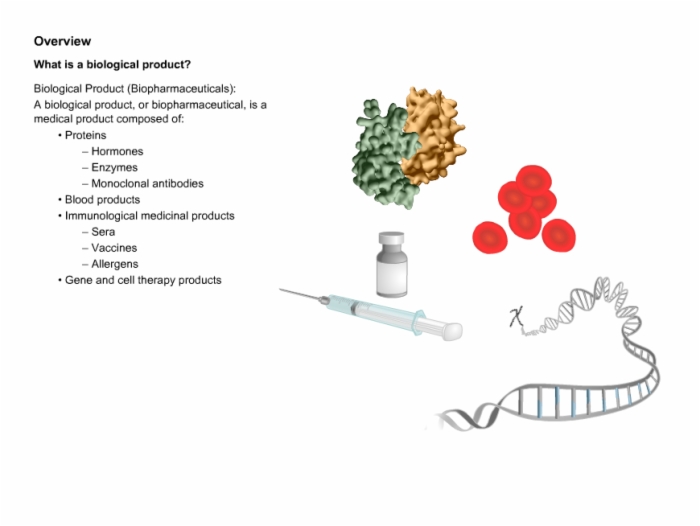

3. Biologics Overview
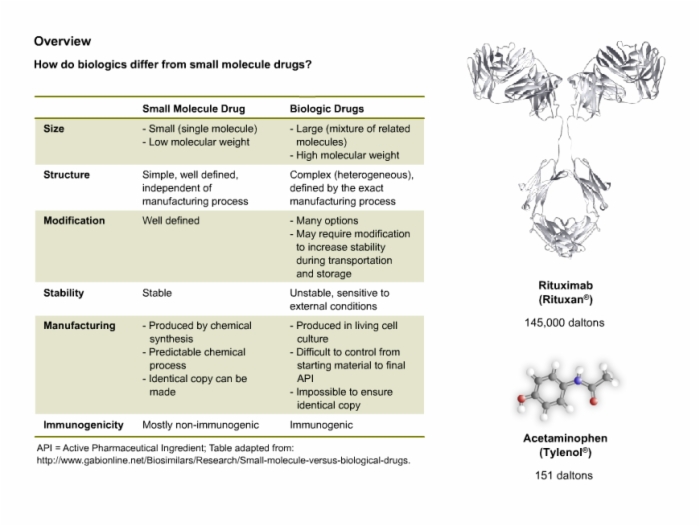

4. Modified Therapeutic Proteins
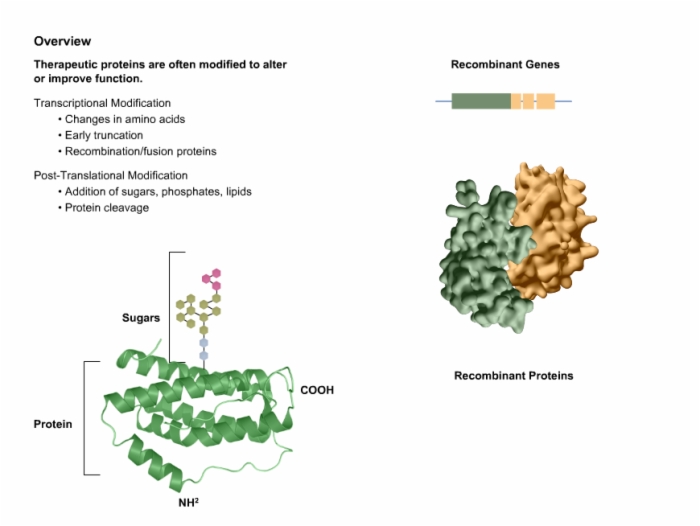

5. Modified Therapeutic Proteins Improve Function
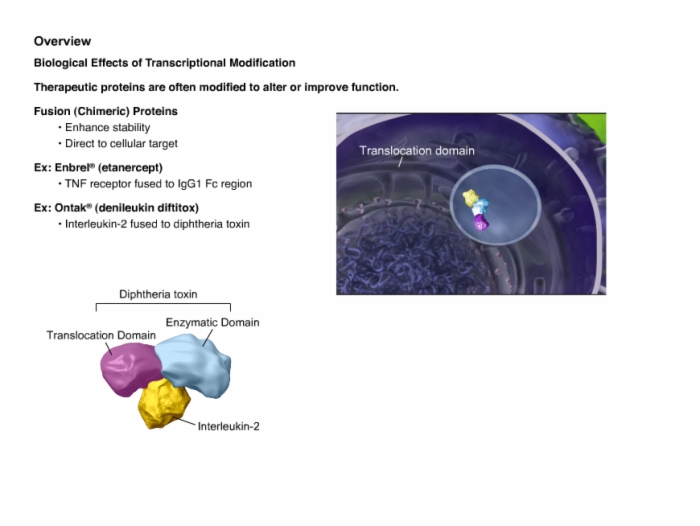

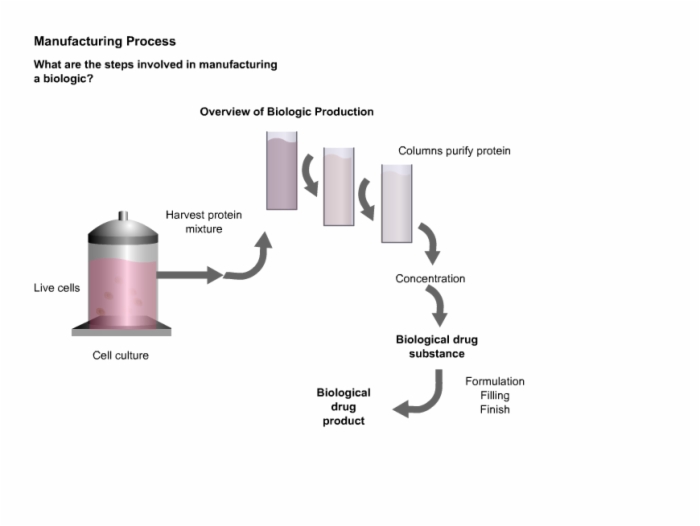
6. Manufacturing Process

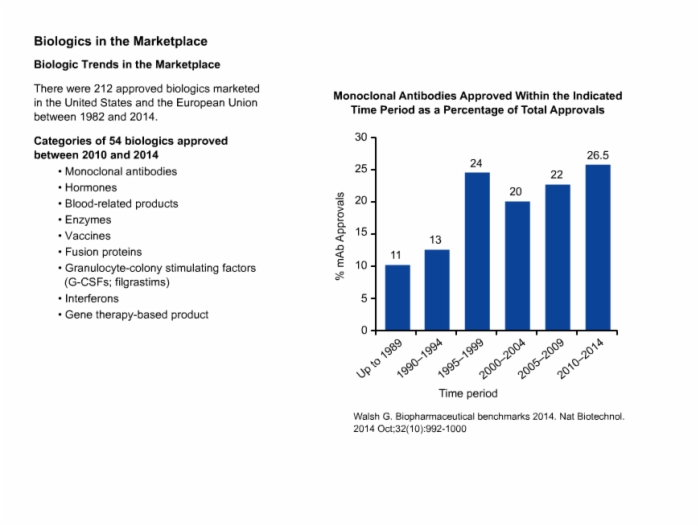
7. Biologics in the Marketplace

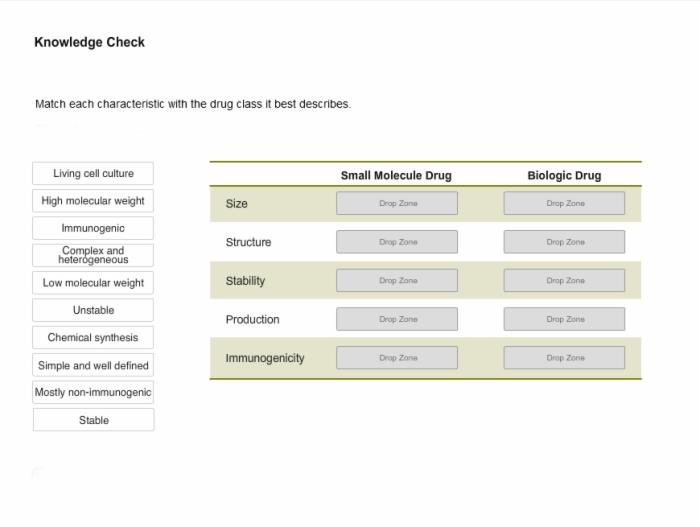
8. Knowledge Check: Characteristics of Small Molecule and Biologic Drugs

9. Knowledge Check: Identifying Biologic Drugs


10. Biosimilars Overview
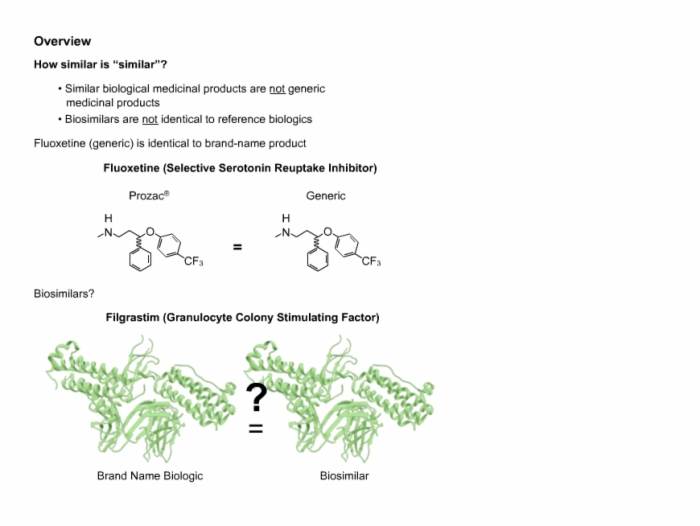

11. Biosimilar Overview and Definition
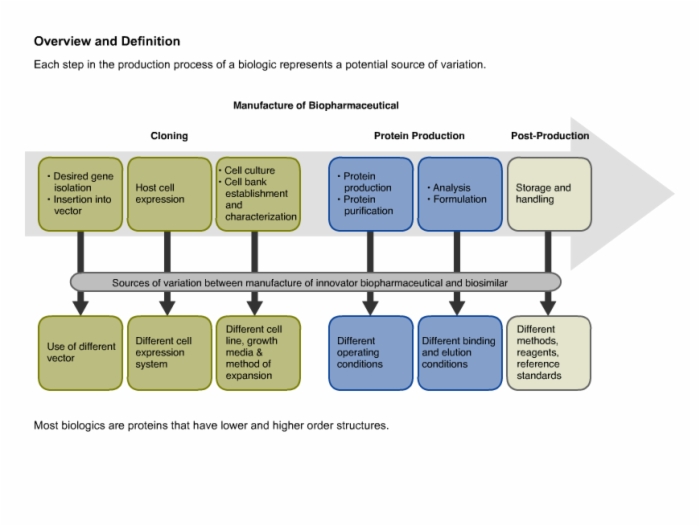

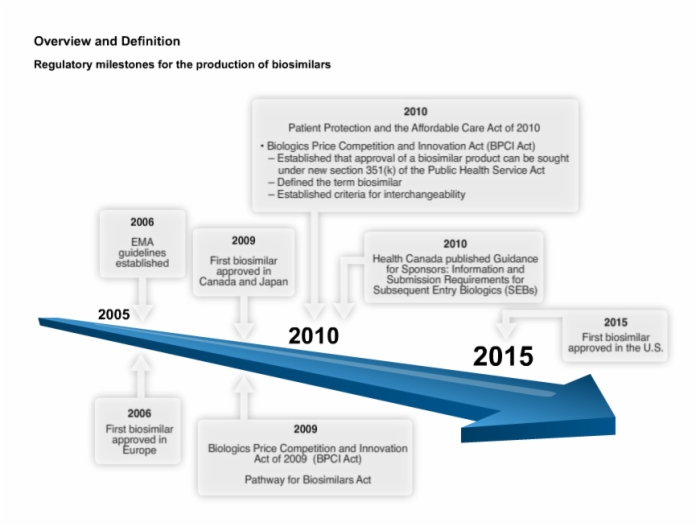
12. Regulatory Milestones and General Requirements for the Production of Biosimilars

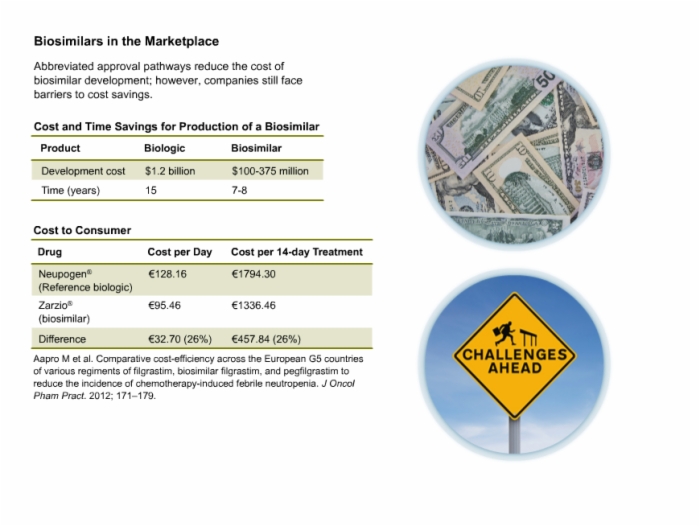
13. Biosimilars in the Marketplace

14. Benefits, Risks, and Patent Life


15. Benefits, Risks, Marketing, and Claims


16. Challenges in U.S. Marketplace

17. Examples of Biosimilars
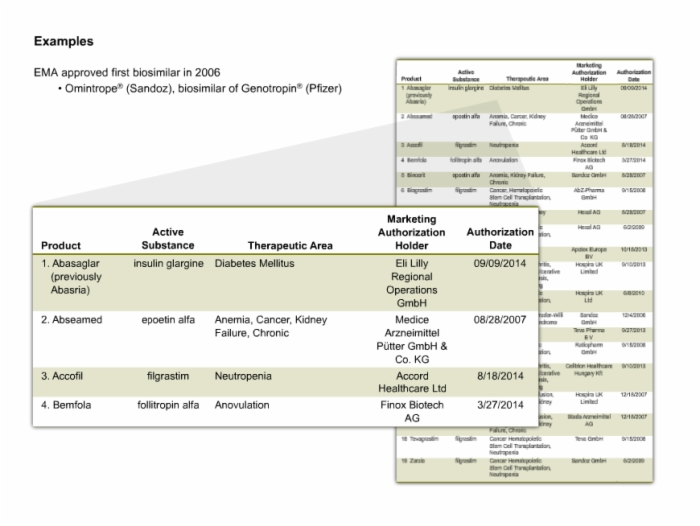

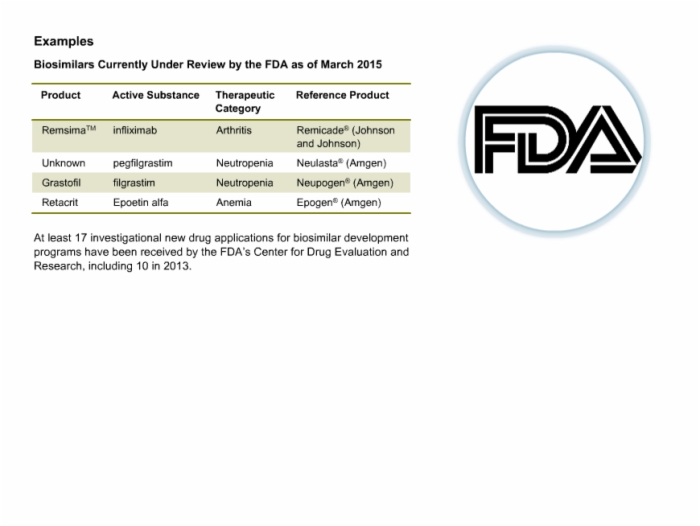
18. Biosimilars Under Review

19. Example of a Biosimilar Not Approved by the EMA


20. Knowledge Check: Biosimilars are...


21. Knowledge Check: Approval of Biosimilars in the EU


22. Knowledge Check: Challenges in Marketing a Biosimilar Drug


23. Approval Process and Regulatory Pathways

24. Reference Product


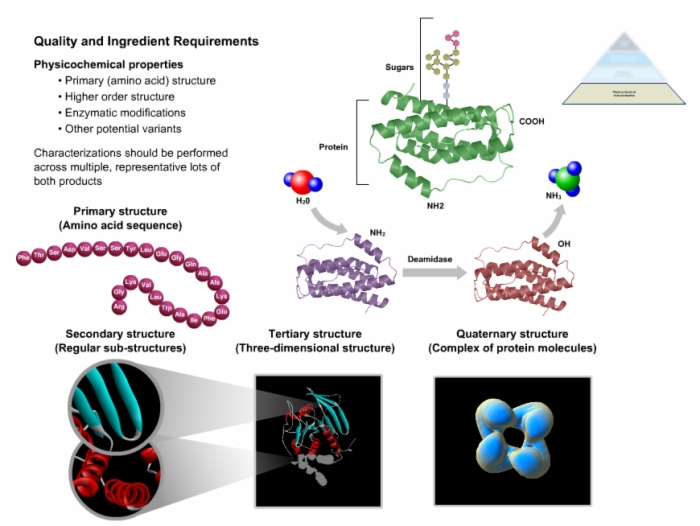
25. Quality and Ingredient Requirements

26. Substitution and Interchangeability


27. Manufacturing and Production
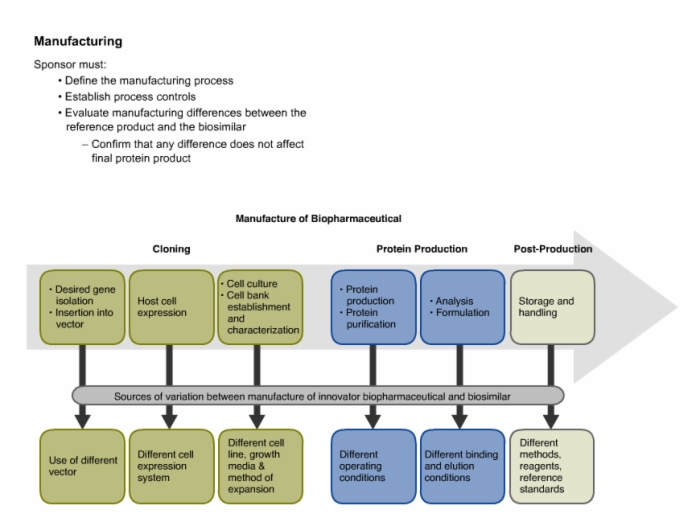

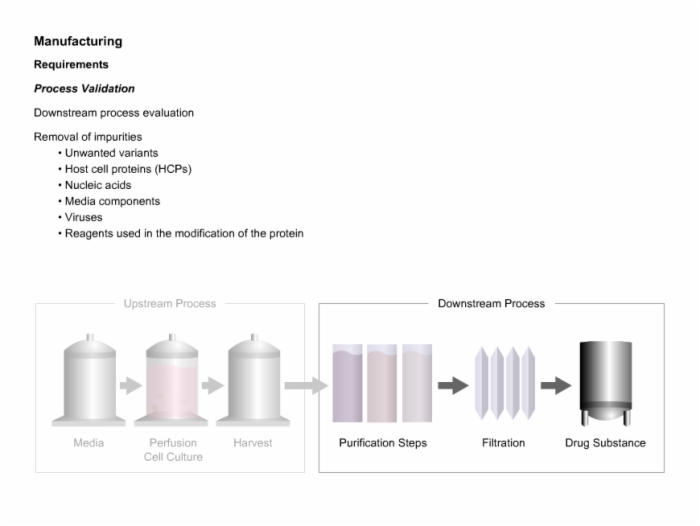
28. Manufacturing Requirements

29. Allowable Differences Between the Biosimilar and the Reference Biologic


30. Evidence Generation: Non-Clinical


31. Evidence Generation: Clinical

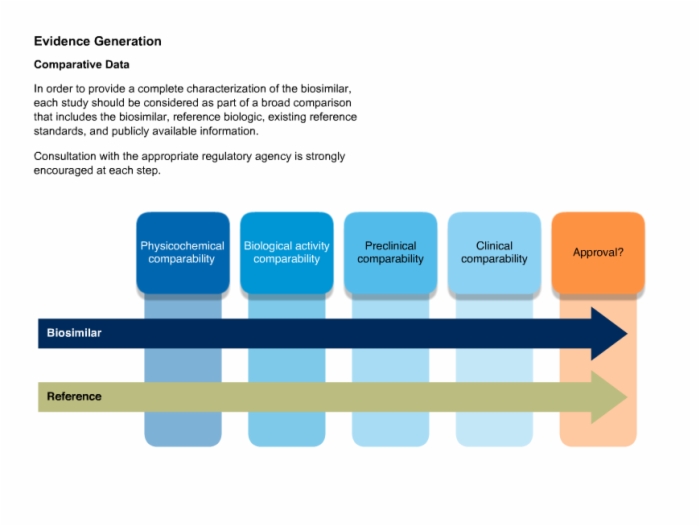
32. Evidence Generation: Comparative Data

33. Hurdles to Approval

34. Pharmacovigilance and Risk Management


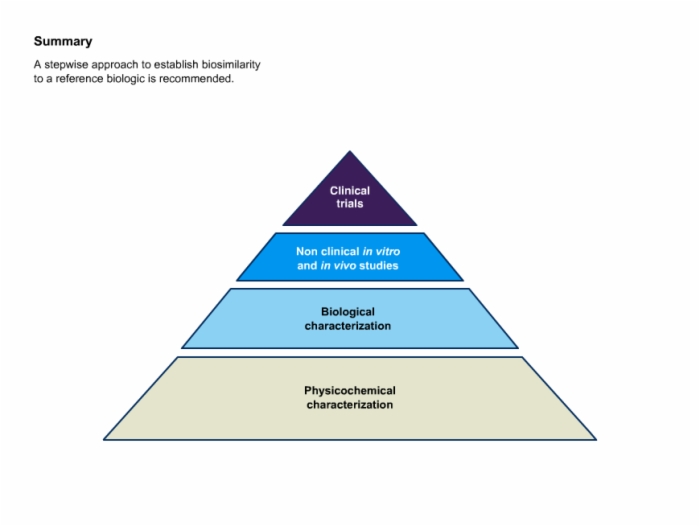
35. Summary: Stepwise Approach to Establish Biosimilarity

36. Knowledge Check: Biosimilar Attributes


37. Knowledge Check: Ligand Binding Assays, Enzymatic Assays, and Functional Assays Testing Use


38. Knowledge Check: Biosimilar Drug Interchangeability


39. Knowledge Check: Roles of a Pharmacovigilance Program


40. Introduction to Country-Specific Definitions and Regulatory Guidelines

41. World Health Organization (WHO)


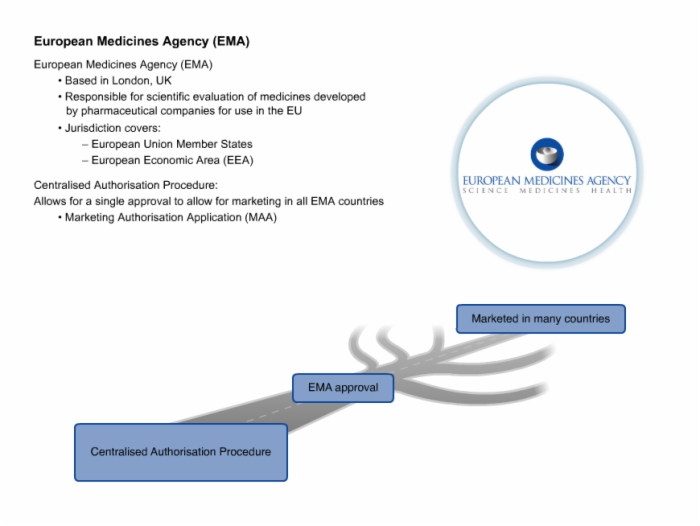
42. European Medicines Agency (EMA)

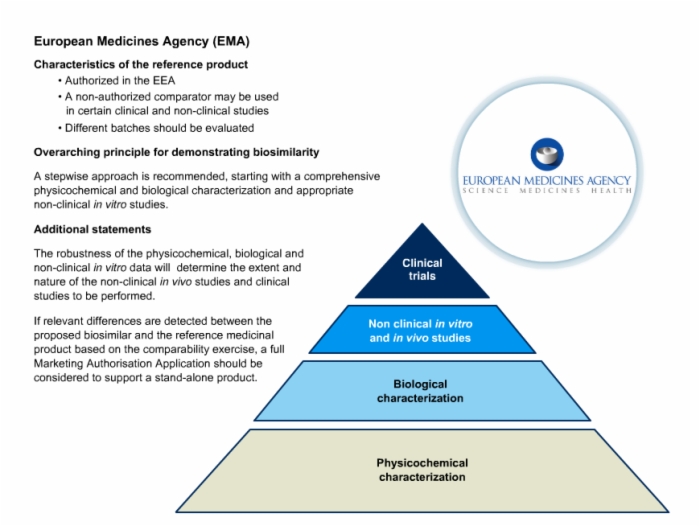
43. European Medicines Agency (EMA) Detailed Requirements

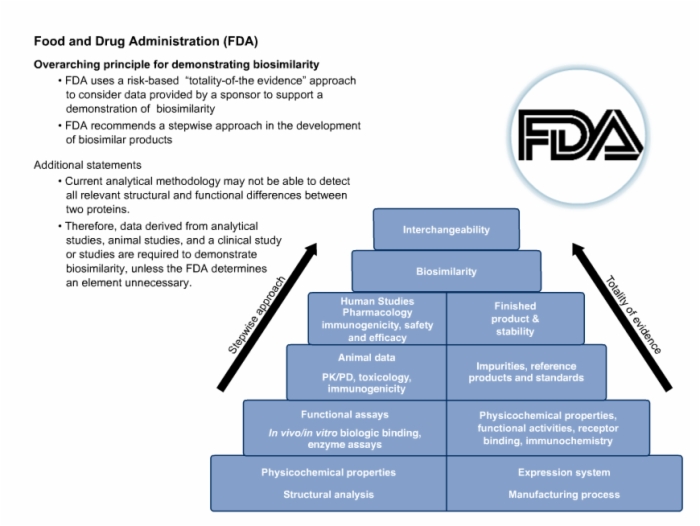
44. Food and Drug Administration (FDA) Detailed Requirements

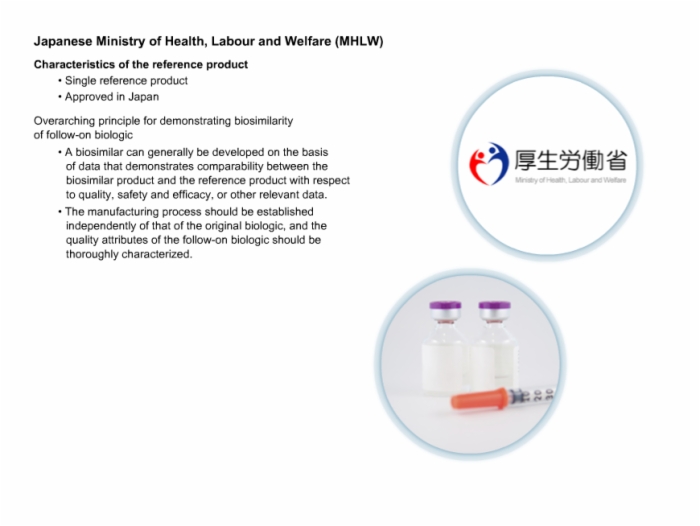
45. Japanese Ministry of Health, Labour and Welfare (MHLW)

46. Health Canada


47. South Korea Ministry of Food and Drug Safety (MFDS)


48. India Ministry of Science and Technology (MST)


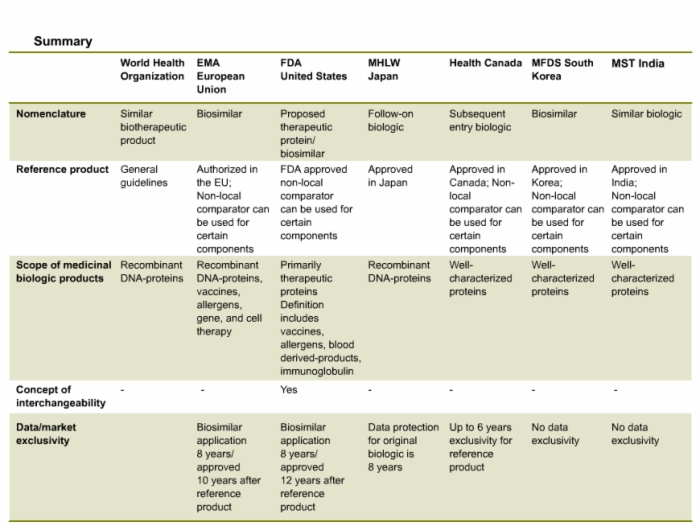
49. Summary of Country-Specific Definitions and Regulatory Guidelines

50. Knowledge Check: Terms Used in Different Countries

51. Knowledge Check: EMA Centralised Authorisation Procedure


52. Knowledge Check: Approving Biosimilars Since 2000


Lesson Biotech: Biosimilars teaches these concepts
Introduction, Introduction to Biosimilars