SMi Source lesson Biotech: Protein Formulation has the following microlearning topics
1. Considerations for Developing a Protein Formulation

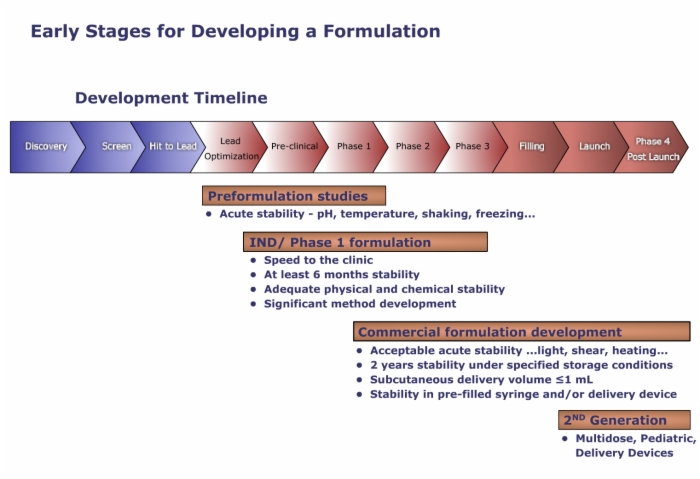
2. Early Stages for Developing a Formulation

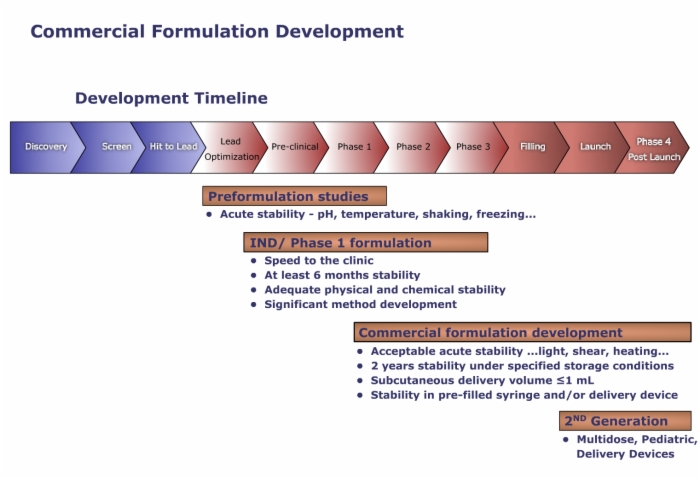
3. Commercial Formulation Development

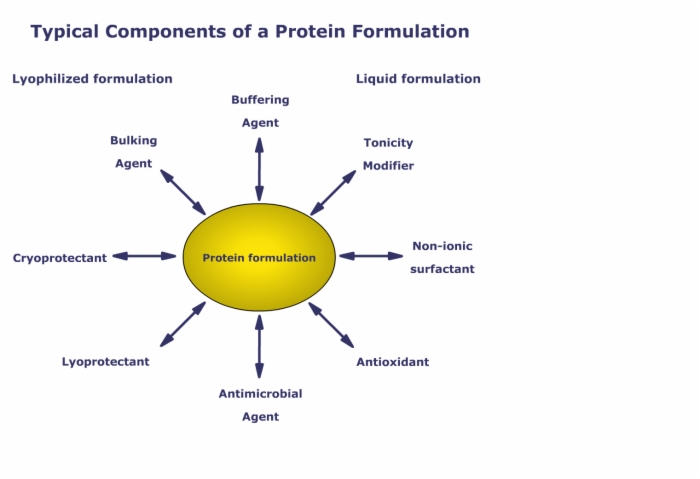
4. Typical Components of a Protein Formulation

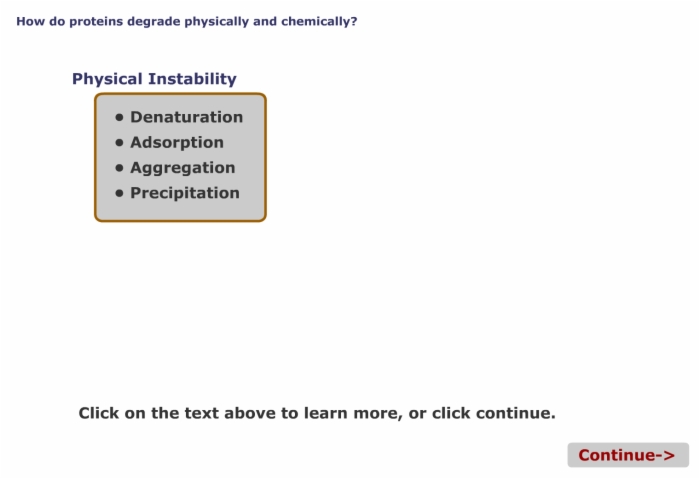
5. How Do Proteins Degrade Physically and Chemically?

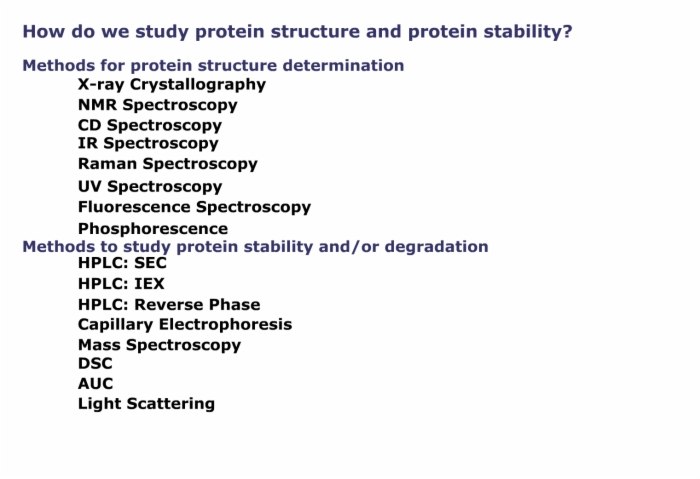
6. How Do We Study Protein Structure and Protein Stability?

Lesson Biotech: Protein Formulation teaches these concepts
Developing a Protein Formulation, Considerations, Introduction
Developing a Protein Formulation, Considerations, Delivery Device
Developing a Protein Formulation, Considerations, Marketing Considerations
Developing a Protein Formulation, Considerations, Development Timeline
Developing a Protein Formulation, Considerations, Resources needed
Developing a Protein Formulation, Considerations, Clinical Indication
Developing a Protein Formulation, Considerations, Patient Population
Developing a Protein Formulation, Considerations, Regulatory Requirements
Developing a Protein Formulation, Considerations, Route of Delivery
Developing a Protein Formulation, Considerations, Dosage Requirements
Developing a Protein Formulation, Considerations, Dosage Form
Developing a Protein Formulation, Considerations, Container Closure
Lesson Biotech: Protein Formulation addresses these key points
Developing a pharmaceutical is a monumental task.
Many challenges exist between getting a drug candidate to a commercial drug product.
The delivery device conveys the formulation from container to patient.
Choice of a particular type of delivery device is largely influenced by dosing requirements and patient convenience.
Having a clear understanding of a commercial formulation’s potential market is critical to its development.
Generally, formulation development starts after the decision is made to start clinical trials since a reliable formulation is required to support preclinical and clinical studies.
There needs to be sufficient quantities of purified protein and pharmaceutically acceptable excipients.
Freeze-drying equipment, sterilized container and closure components and an aseptic fill and finishing environment are required.
Analytical instruments required for structural analyses.
Facilities with controlled temperature, controlled light exposure, controlled relative humidity, and devices to provide controlled agitation are necessary to perform stability studies.
Human resources - Efficient and effective utilization of these resources is not possible without input from knowledgeable and skilled personnel from a variety of departments.
Questions that need to be answered related to the clinical indication include:
- Site of treatment (self-administration, office visit, hospital)
- Method of delivery
- Concomitant medication
- Are there similar medications?
The target patient population needs to be clearly defined.
Information necessary for designing formulation studies includes: patient age, strength, tolerability, capacity to manipulate delivery devices, and possible sensitivity to excipients.
Consideration of regulatory requirements in the formulation development process is both beneficial to patients and critical for regulatory approval of the commercialized product.
Protein therapeutics are easily degraded by the digestive system, so delivery by injection or infusion is usually required.
- Injectables (IV, SC, IM, IP, ICV, IT, IO)
- Topical
- Inhalation
- Oral
Dosage requirements need to be considered in order to achieve the intended therapeutic action.
The stability, physical properties, and reconstitution of the product must be considered when choosing a dosage form.
Considerations in choosing the type of container include: product contact with the container material, leachates, breakage, light sensitivity, and moisture penetration. These factors can affect the stability of the therapeutic protein, so extensive studies must be conducted to assure that the container and closure are compatible with the protein formulation.
Lesson Biotech: Protein Formulation is built from these main references. Log into SMi Source for a complete list and details.
Carpenter, J.F. and Manning, M.C., Rational Design of Stable Protein Formulations: Theory and Practice (Springer, 2002), p. 3-20.
ANSEL'S PHARMACEUTICAL DOSAGE FORMS AND DRUG DELIVERY SYSTEMS By Loyd V. Allen, Nicholas G. Popovich, Howard C. Ansel, 2004, Lippincott Williams& Wilkins p641.