SMi Source lesson Diabetes: Pathology Complications - Hyperglycemia and Glucotoxicity has the following microlearning topics
1. Underlying Mechanisms of Chronic Complications

Lesson Diabetes: Pathology Complications - Hyperglycemia and Glucotoxicity teaches these concepts
Diabetes, Underlying Mechanisms of Chronic Complications, Hyperglycemia and Glucotoxicity
Lesson Diabetes: Pathology Complications - Hyperglycemia and Glucotoxicity addresses these key points
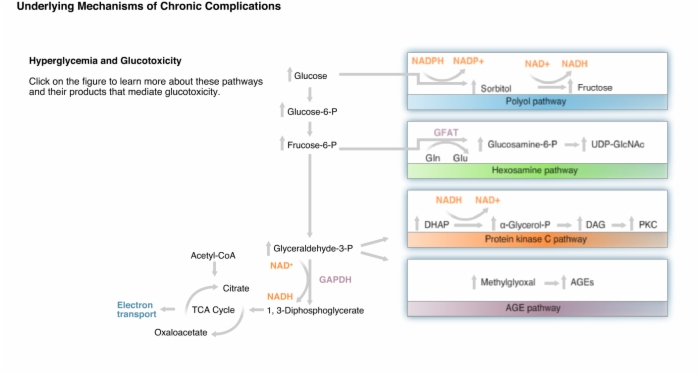
Normoglycemia:
- Glucose is oxidized by glycolytic pathway and tricarboxylic acid cycle to form NADH and FADH2.
- These electron donors feed into the mitochondrial electron transport system to generate ATP by oxidative phosphorylation.
Hyperglycemia:
- Some of the glucose is diverted through these and other pathways forming molecules which can damage cells such as endothelial cells lining blood vessels.
- Aldose reductase has a relatively low affinity for glucose, but in hyperglycemia, some glucose is converted to sorbitol, which is oxidized to fructose.
- Increased flux of glucose through the polyol pathway increases intracellular NADH, decreases NADPH, and depletes the natural antioxidant, glutathione (GSH), exacerbating potential oxidative stress.
- Excess fructose-6-phosphate can be diverted from glycolysis to the hexosamine pathway where it is converted to glucosamine-6-phosphate.
- UDP serves as a carrier of N-acetylglucosamine for the glycosylation of proteins.
- One protein that is glycosylated is transcription factor Sp1, which then becomes more active.
- Sp1 activates transcription of genes including those for prothrombotic factors, PAI-1 and TGFβ.
- Dihydroxyacetone phosphate can be reduced and acylated to form diacylglycerol, a lipid cellular second messenger.
- Diacylglycerol activates beta and delta isoforms of protein kinase C.
- Protein kinase C activation has multiple consequences in the endothelium.
- Many of the deleterious effects involve alteration in the levels of vasoactive molecules such as nitric oxide and vascular endothelial growth factor.
- Protein kinase C actvation also leads to increases in fibrogenic proteins such as TGFβ, collagens, and fibronectin.
- Reactive dicarbonyls are formed from glucose and intermediates of glycolysis.
- Glycation of intracellular proteins can disrupt normal cellular processes.
- Modification of extracellular matrix proteins causes abnormal interactions with other matrix proteins and with integrins on neighboring cells, disrupting normal intercellular communications.
- Glycation of plasma proteins allows them to interact with AGE receptors on endothelial cells, mesangial cells, and macrophages.
- Binding to AGE receptors activates NF-κB and alters gene expression.
- All of these can activate cascades of factors that cause cellular and tissue damage.
- Increased glucose oxidation and electrons flowing into the electron-transport chain creates an abnormally high mitochondrial membrane potential that inhibits electron transport at complex III.
- This allows coenzyme Q to donate electrons to oxygen to form the free radical superoxide.
- Free radicals induce DNA strand breaks and in turn activate the enzyme poly(ADP-ribose) polymerase or PARP.
- Glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase is ADP-ribosylated and inactivated.
- All intermediates upstream of glyceraldehydes-3-phosphate accumulate and are diverted to these pathways that mediate hyperglycemic damage.