SMi Source lesson Drug R&D: Clinical Statistics in Clinical Trials has the following microlearning topics
1. The Use of Statistics in Clinical Trials and Scientific Hypothesis Testing

2. Hypotheses


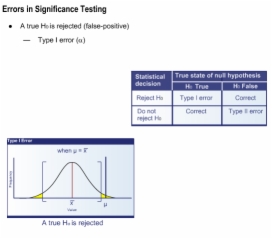
3. Why is it Important to Control Type I Error?

4. What is the Intent to Treat Principle?


5. The Role of Interim Analysis


6. How Are Sample Size Calculations Made?

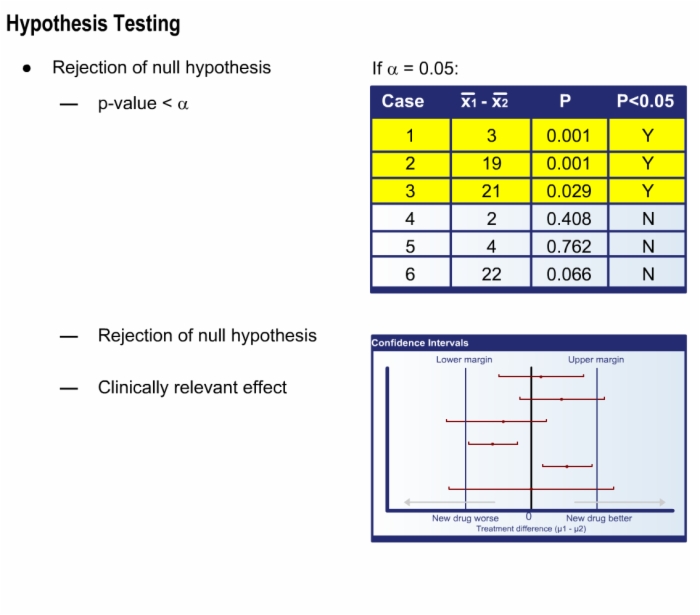
7. How to Interpret Study Results?

8. Summary


Lesson Drug R&D: Clinical Statistics in Clinical Trials teaches these concepts
Use of Statistics in Clinical Trials, Introduction, The Use of Statistics in Clinical Trials and Scientific Hypothesis Testing
Lesson Drug R&D: Clinical Statistics in Clinical Trials addresses these key points
The Use of Statistics in Clinical Trials
After completing this module, you should be able to:
- Formulate a null hypothesis from the study objective
- Distinguish between type I and type II errors
- Estimate the effect multiplicity has on the magnitude of type I errors
- Define the intent-to-treat principle
- Explain the role of interim analyses
- Estimate how significance level and other design parameters impact sample size
- Interpret statistical tests
- Distinguish statistical significance from clinical relevance
Scientific Hypothesis Testing
- Key element of clinical trials
- Statistical evaluation of therapeutic intervention