SMi Source lesson Drug R&D: Combination Product Regulatory Requirements has the following microlearning topics
1. Welcome

2. Learning Objectives


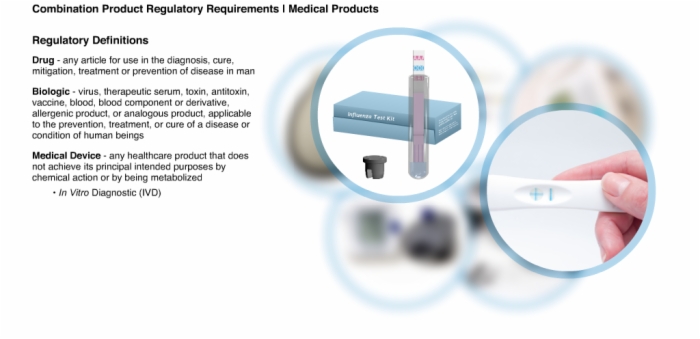
3. Regulatory Definitions

4. Regulatory Definitions: Combination Product


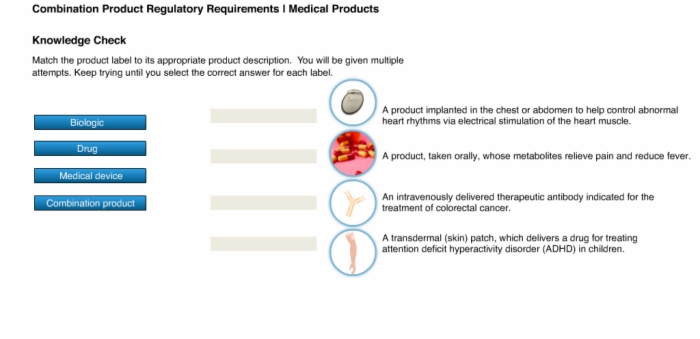
5. Knowledge Check: Product Descriptions

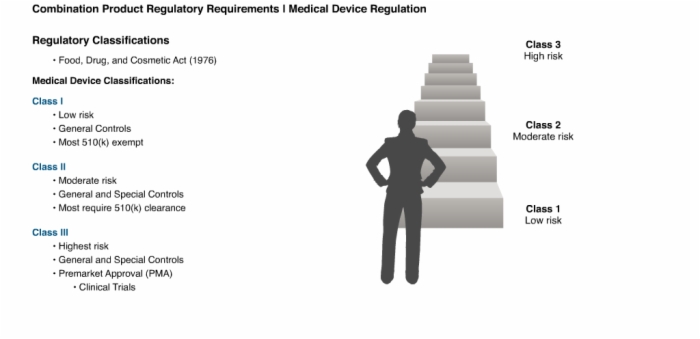
6. Regulatory Classifications

7. Regulatory Classifications: EU Regulatory Requirements

8. Regulatory Classifications: Class II Devices


9. Class II Devices: 513(g)


10. Regulatory Classifications: Class II Devices

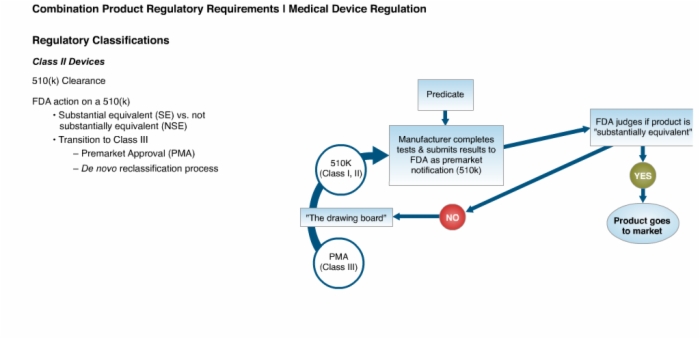
11. Class II Devices: 510(k) Clearance

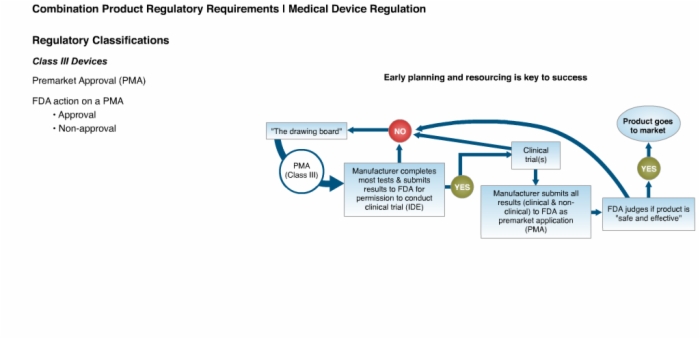
12. Class III Devices

13. Knowledge Check: Medical Devices


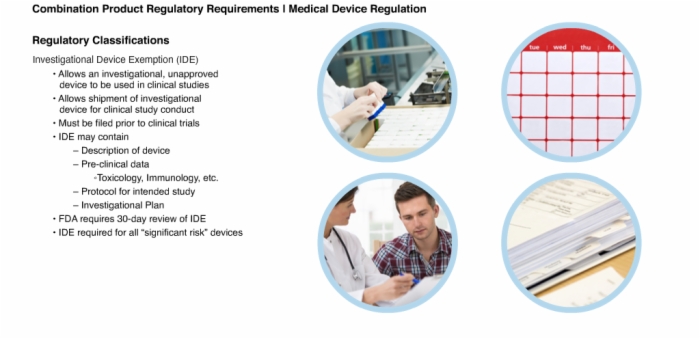
14. Regulatory Classifications: Investigational Device Exemption (IDE)

15. General Controls


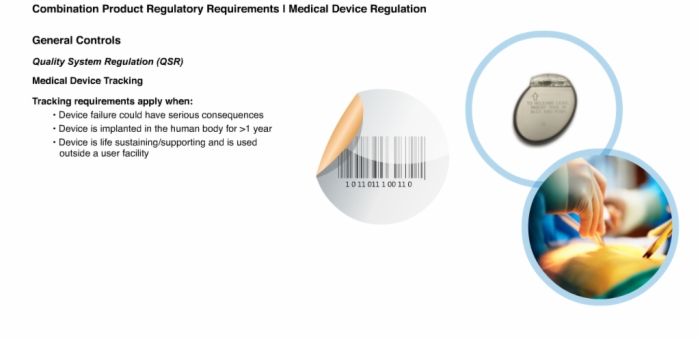
16. General Controls: Quality System Regulation (QSR)


17. General Controls: Quality System Regulation (QSR)

18. European Union Requirements (CE mark)

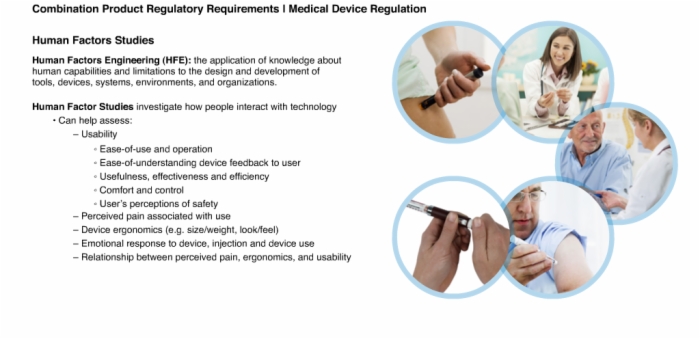
19. Human Factors Studies

20. Knowledge Check: Components of a Quality System


21. Knowledge Check: Approved Medical Devices in the EU


22. Knowledge Check: Classes of Medical Device


23. Biological Product Regulation


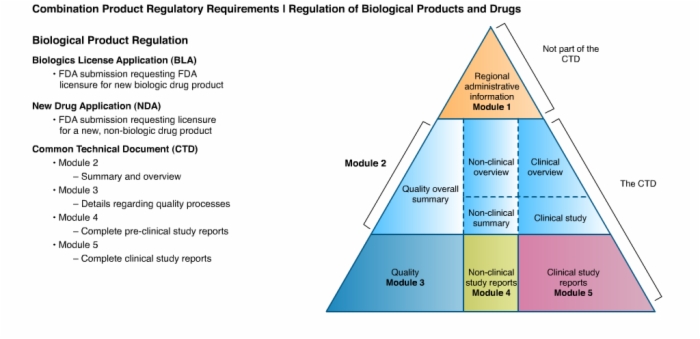
24. Biological Product Regulation: Biologics License Application (BLA), New Drug Application (NDA), Common Technical Document (CTD)

25. Drug Regulation, Clinical Studies, CDT, and CMC


26. Drug Regulation: European Registration International Conference on Harmonization (ICH)

27. Knowledge Check: Most Common Submission Method


28. Knowledge Check: Investigational New Drug Application


29. Knowledge Check: New Drug Application (NDA)


30. Knowledge Check: Drug Master File (DMF)


31. Knowledge Check: CMC Document


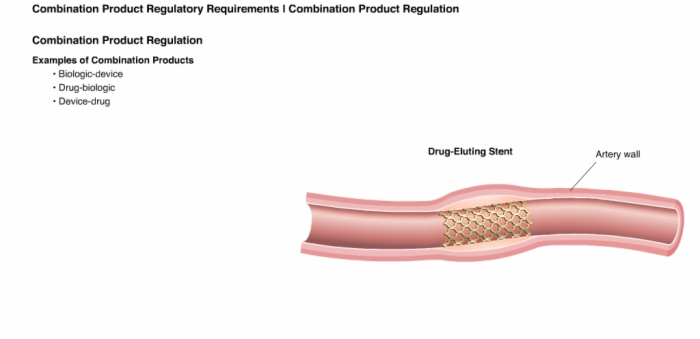
32. Combination Product Regulation

33. Combination Products: Approval as Drug or Device

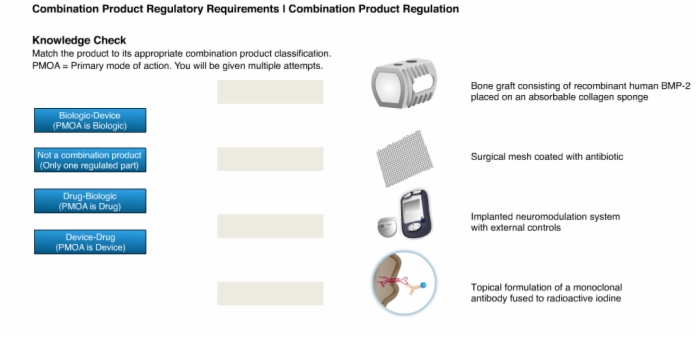
34. Knowledge Check: Combination Product Classification

35. Combination Products: Approval as Drug or Device?


36. Combination Products: One or Two Submissions?


37. Combination Products: One or Two Reviewers?


38. Quality System Requirements/cGMPs

39. Post-Marketing


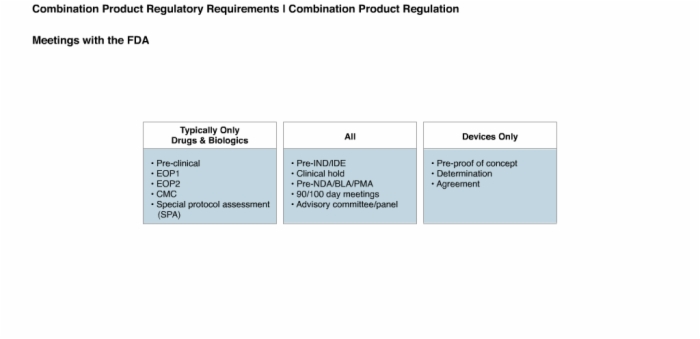
40. Product Disposal and Meetings with the FDA

41. Knowledge Check: FDA Regulations


42. Medical Products, Medical Device Regulation, Biological & Drug Product Regulation and Combination Product Regulation