SMi Source lesson Diabetes: Insulin Biosimilars has the following microlearning topics
1. Introduction

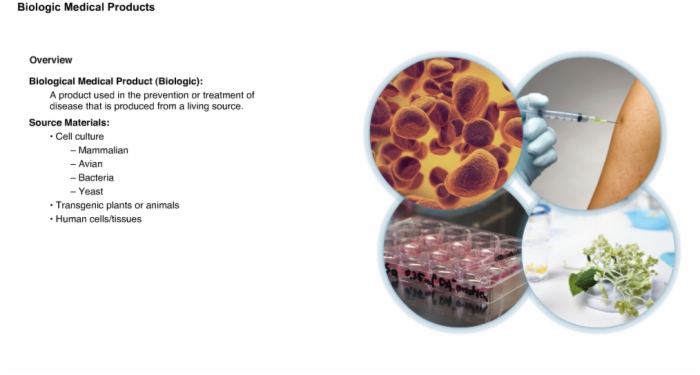
2. Overview: Biologic Medical Products

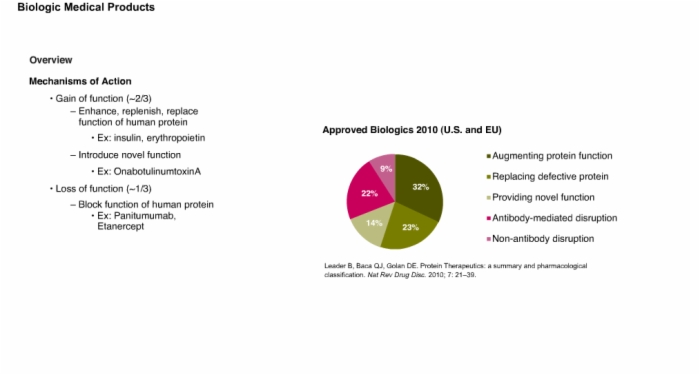
3. Overview: Regulatory Definitions and Mechanism of Action

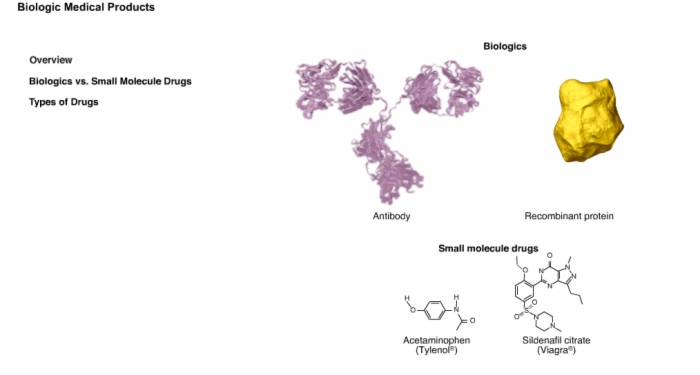
4. Overview: Biologics vs. Small Molecule Drugs

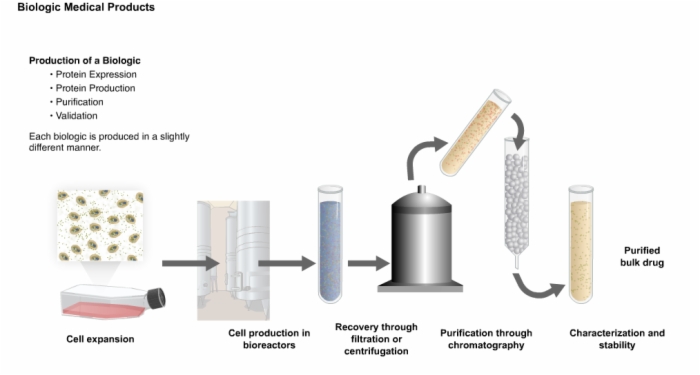
5. Production of a Recombinant Biologic

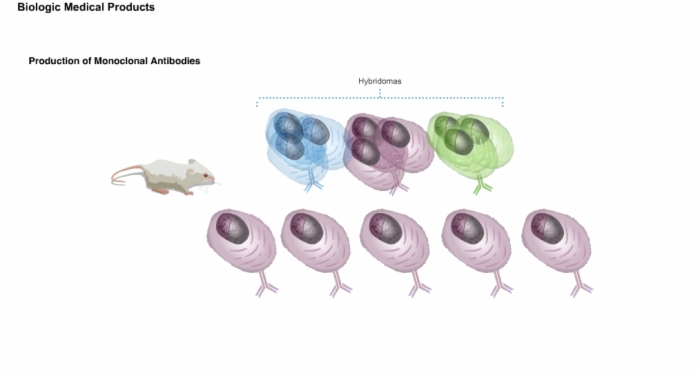
6. Production of Monoclonal Antibodies

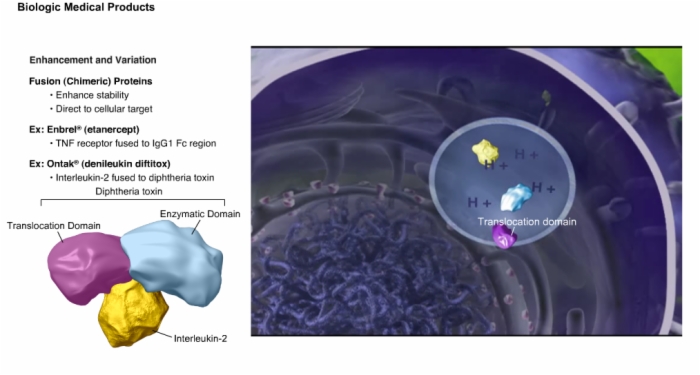
7. Enhancement and Variation

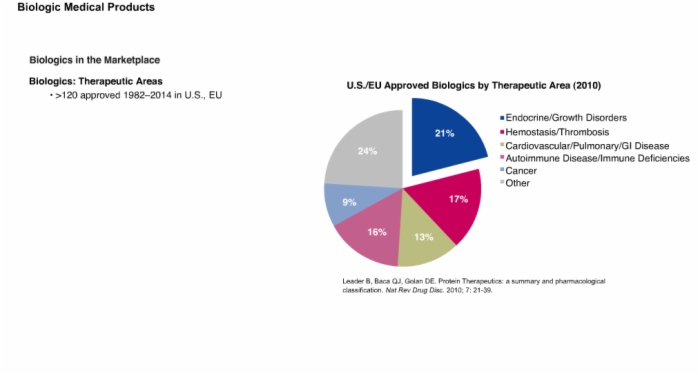
8. Biologics in the Marketplace

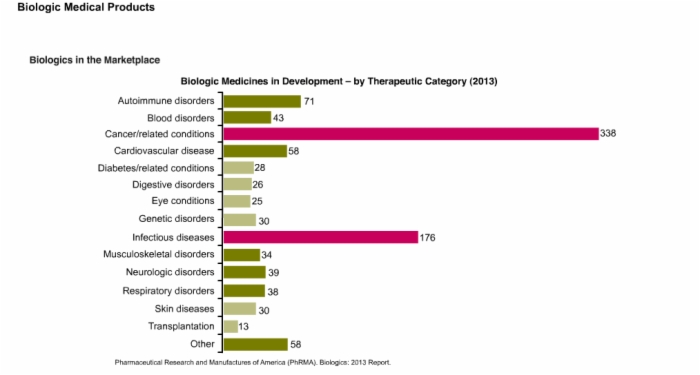
9. Patent Cliff and Biologic Medicines in Development

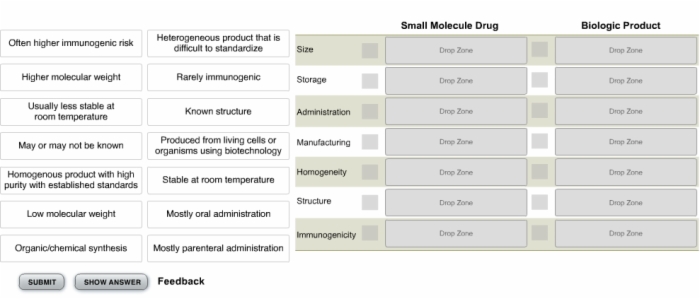
10. Knowledge Check: Small Molecule Drug vs. Biologic Medicinal Product
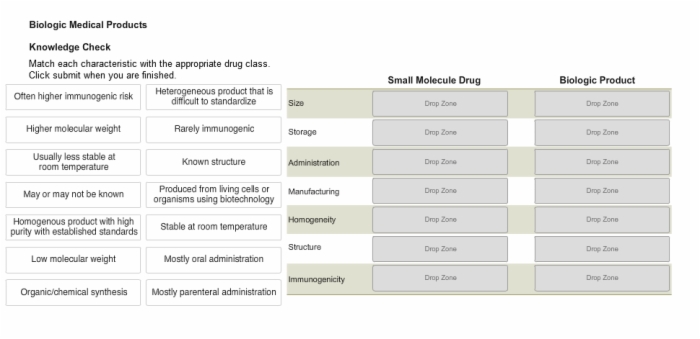

11. Knowledge Check: Characteristics of Specific Drug Classes

12. Knowledge Check: Example of a Post-Translational Modification


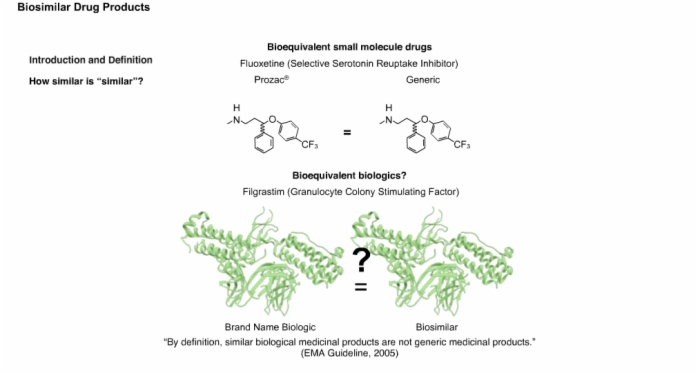
13. Introduction and Definition: Biosimilars

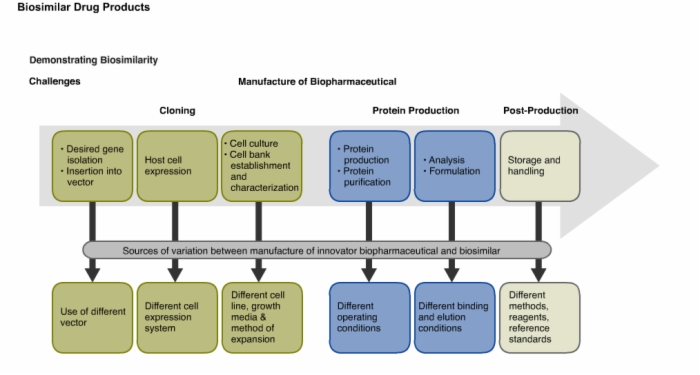
14. Demonstrating Biosimilarity: Challenges

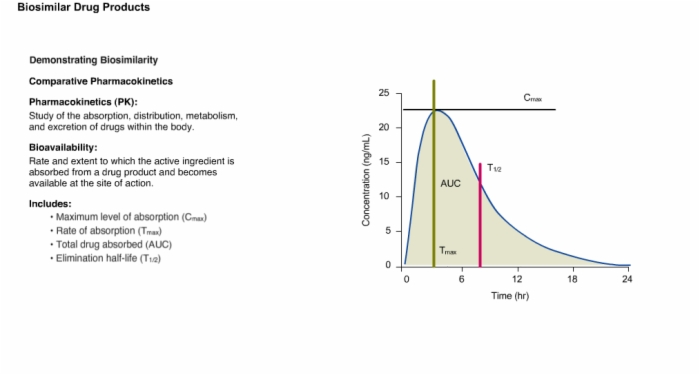
15. Demonstrating Biosimilarity: Comparative Pharmacokinetics

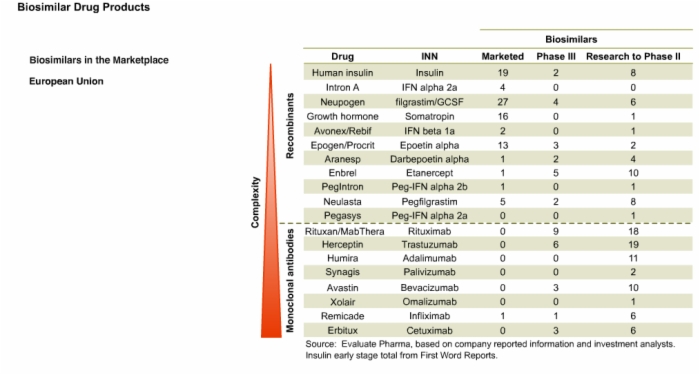
16. Biosimilars in the Marketplace and Drug Development in the U.S.

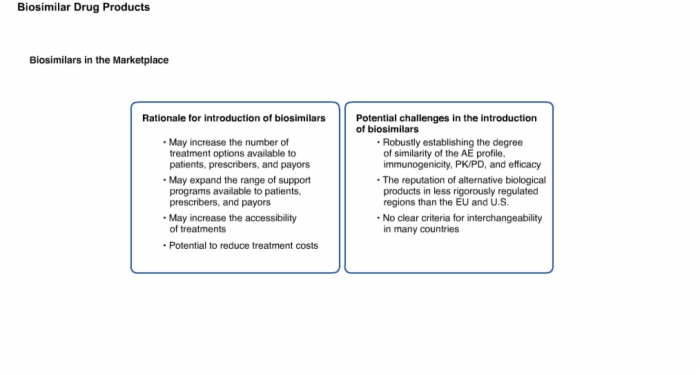
17. Biosimilars in the Marketplace

18. Knowledge Check: Pharmacokinetic Parameters


19. Knowledge Check: Biological Products


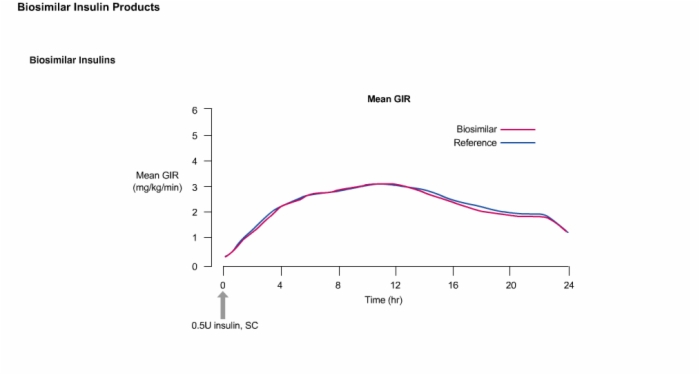
20. Biosimilar Insulins

21. Knowledge Check: 2012 EMA Draft Guideline on Development of Biosimilar Insulins


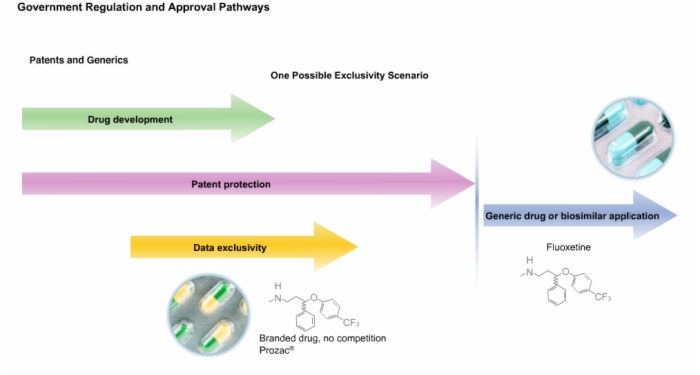
22. Drug Patents and Generics

23. European Medicines Agency (EMA)


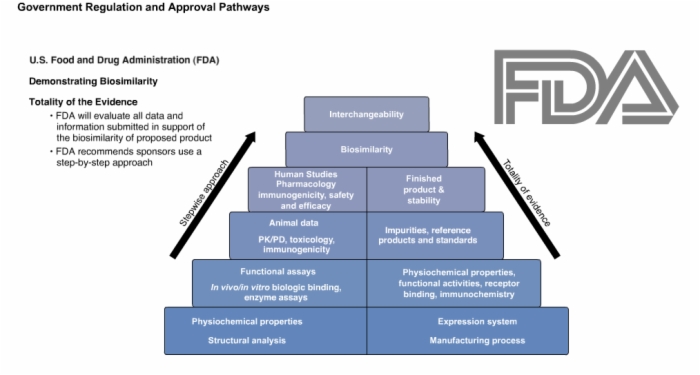
24. U.S. Food and Drug Administration

25. U.S. Food and Drug Administration: Regulatory Oversight


26. Knowledge Check: U.S. and U.K. Regulation of Biosimilars


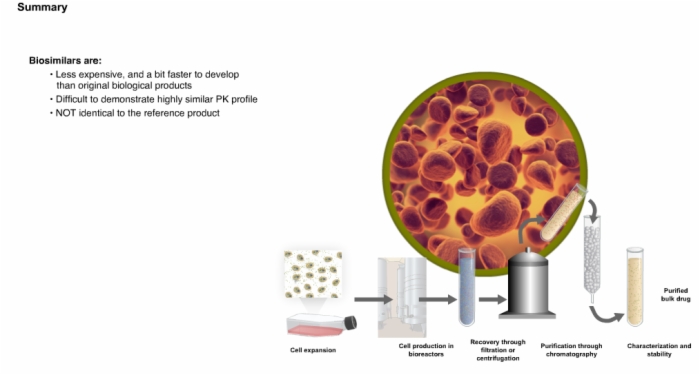
27. Summary: Biologics and Biosimilars