

shift in clinical trial protocol
training and compliance.

Powered by ScienceMedia MindFlowTM, the Science of Learning.

Powered by ScienceMedia MindFlowTM, the Science of Learning.

Powered by ScienceMedia MindFlowTM, the Science of Learning.


Powered by ScienceMedia MindFlowTM, the Science of Learning.

shift in clinical trial protocol
training and compliance.



Powered by ScienceMedia MindFlowTM, the Science of Learning.

shift in clinical trial protocol
training and compliance.
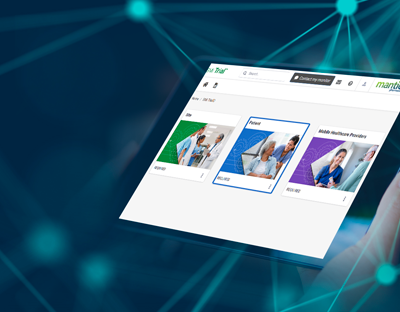
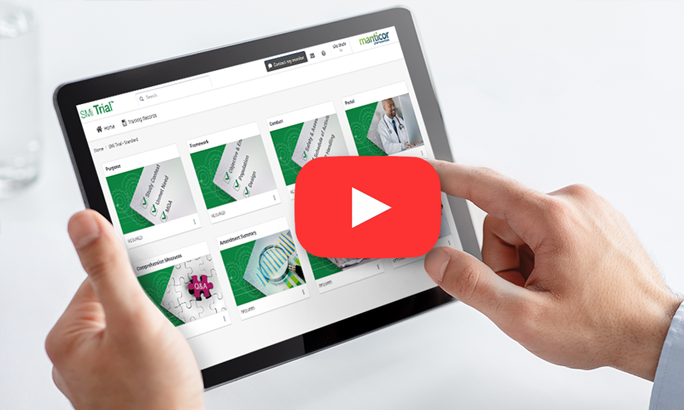
SMi Trial is a user-friendly, integrable cloud-based mobile platform with customized content.
We empower biopharmaceutical and medical device companies and Contract Research Organizations (CROs) to embrace the science of learning to:
Protocol Training
Elevate and standardize site performance with custom multimedia training targeted to high-risk areas of your protocol.
Just-In-Time Access
Fight the "forgetting curve" with online protocol and medical distance learning to ensure your study team is always prepared with mobile-enabled access from any device, anytime, anywhere.
Interactive Schedule of Activities
Centralize key study procedure instructions with an interactive visit-specific guide.

Protocol Amendment Management
Deploy, track, and re-train staff on protocol amendments throughout the lifetime of your study.
Audit-Ready Reports
Report the training completion of your study team with printable, audit-ready training records.
Study Updates
Share key lessons with custom updates targeting knowledge gaps discovered during the execution of the study.
Virtual Investigator Meetings and Site Initiation Visits
Reimagine traditional IMs and SIVs with remote pre-training, self-paced micro learning, standardization, and assessments. Access our webinar to learn more.

Real-Time Analytics
Evaluate site staff competency in real time using role-based analytics on training status and comprehension.
Integrated Assessments
Identify knowledge gaps and activities at high risk of deviation and ensure compliance with ICH E6(R2) using protocol-specific training assessments.

SMi Trial users can visualize the protocol in action, bringing it to life
The magic is in our individualized protocol learning journey for each user who can go at their own pace to ensure knowledge retention and compliance with complex protocols. Based on our ScienceMedia MindFlowTM methodology that harnesses cognitive and behavioral learning theories, SMi Trial gives clinical trial training operations leaders the power to de-risk trials, increase diverse participant retention, streamline compliance documentation and deliver unparalleled ROI.
- Ideal for sponsors of multi-site trials grappling with ongoing training of complex and changing protocols and staff turnover issues.
- Proven its effectiveness in recent clinical trials, where it achieved record-breaking usage of over one million views in the first year, demonstrating its ability to engage site staff and ensure protocol compliance.
- Replacing traditional protocol training decks or outdated systems with a highly effective multimodal learning experience.
SMi Trial users can visualize the protocol in action, bringing it to life
The magic is in our individualized protocol learning journey for each user who can go at their own pace to ensure knowledge retention and compliance with complex protocols. Based on our ScienceMedia MindFlowTM methodology that harnesses cognitive and behavioral learning theories, SMi Trial gives clinical trial training operations leaders the power to de-risk trials, increase diverse participant retention, streamline compliance documentation and deliver unparalleled ROI.
- Ideal for sponsors of multi-site trials grappling with ongoing training of complex and changing protocols and staff turnover issues.
- Proven its effectiveness in recent clinical trials, where it achieved record-breaking usage of over one million views in the first year, demonstrating its ability to engage site staff and ensure protocol compliance.
- Replacing traditional protocol training decks or outdated systems with a highly effective multimodal learning experience.
Get in touch with us
Let's talk about transforming your stakeholders' clinical mastery
