ScienceMedia and Halloran Consulting Group (Halloran) recently collaborated during a Halloran-hosted CORE Retreat - a Clinical Operations Retreat for Executives - in the Boston area. The connection between these companies is simple. They understand the proven need to provide results-driven and multi-media format training to sponsors operating clinical trials across all phases of the study. ScienceMedia's PhDs and instructional designers produce the training modules, and Halloran's executives and consultants know the pain points sponsors face that make such training necessary as a part of their overall quality management strategy.
During the CORE East event, ScienceMedia hosted a panel on "Training and Technology: Challenges Around Continuous Education and Site Engagement," led by Malachi Bierstein, Vice President of Global Sales and Marketing at ScienceMedia, Philip Bedrin, Director of Medical and Clinical Learning Strategy & Solutions at ScienceMedia, and Michael McKay, Global Trial Leader at the Janssen Pharmaceutical Companies of Johnson & Johnson.
The room was filled with Clinical Operations Executives eager to learn how to move clinical trial training out of the dark ages - ahem, a single and robust PPT during study-start up - and into a more contemporary, bite-sized, multi-format solution that is fit for all those involved in a clinical trial across the entire lifecycle. The benefits of this training approach are vast. They include, but certainly aren't limited to, streamlining enrollment, reducing screen failures, preventing protocol deviations, standardizing compliance, ensuring clinical data quality, and completing studies earlier, faster, and more efficiently. Clinical trials are expensive to run, and they're even more expensive if errors due to a lack of proper training and quality management prolong the trial's length.
This article will reveal the value of such training, why the clinical research community is due for a change, a few successful examples, and suggestions to take back to your organization for further consideration.
Clinical Research Community is Due for Change - Moving Out of the Dark Ages
Clinical trial training hasn't evolved over the years, and unfortunately, doesn't account for the growing complexity of clinical operations. 'Unfortunately' is used intentionally because current training processes place a heavy burden on the sponsor to train, develop and provide materials, oversee the process, and provide assessment checks on a continual basis. And it's not a beneficial process for those receiving the training and for the health of the trial. When we think about current training, it often looks like this: sponsors train their trial site staff with a lengthy PowerPoint presentation followed by a bolt-on learning workshop, and then that's a wrap. Current approaches throw a lot of information to employees upfront. The major downside of this approach is that it doesn't account for how different people learn, maintain information, and how they need support and training throughout each phase of the trial to ensure widespread compliance.
Part of the reason training isn't fully maximized is that it's often an afterthought and is a bolt-on addition to a study start-up. The problem with a lack of planning and training execution is, for example, a couple of years down the road once that trial has progressed into a phase II study, the sponsor will likely find major deviations, quality, and data problems that stem from a lack of training. Those are big problems.
It's our point of view that at least six months of preparation to design a training program is adequate. Designing a training program that addresses all those involved in the trial operations - and even the patients - takes significant consideration. Michael McKay from the panel mentioned, "if you are thinking about upping your training game, think in advance. You can't just throw in training. Think about a multi-media approach, and that takes a lot of planning." That sentiment is echoed.
From the site perspective, it's our understanding this is their preferred approach:
Training for complex components of the trial protocol as early as possible
Removal of foundational content where competency already exists
Acknowledgment that not all employees learn the same way
Awareness that not all employees prefer to receive their training via one source
Since site staff are working in a hybrid environment, SMi Trial is also mobile-enabled.
 ©ScienceMedia 2021. SMi Trial mobile enablement.
©ScienceMedia 2021. SMi Trial mobile enablement.
Then ScienceMedia takes that further. The philosophy behind the training modules is a deep-rooted understanding that a multi-media format broken down into short microlearning topics with knowledge checks increases retention. This type of learning allows study teams and site staff to reference something quickly to remain in lockstep with the protocol.
Enabling Patient-Facing Training for an Enhanced Patient Experience
Patients also have to navigate a significant amount of complexity during their clinical trial participation, and like site staff, they too are an essential customer in the trial experience. This dynamic propelled ScienceMedia to also offer proper training to patients.
Patients require different information during each unique phase of the trial. During the research or study startup phase, they want to know what's required of them so they can make an educated decision to participate, and they also need information on how the site operates from a logistics perspective to understand what is feasible. Additionally, they need to know the 'why' - why are they participating? Ultimately, patients want to know how a trial is going to help them and increase their odds of living. Once that core foundation is set, and because there is a deluge of information shared with them, they will benefit tremendously from smaller components of information shared throughout the trial as opposed to heavy information all at once upfront.
The teams at ScienceMedia see that patient-facing training is at the forefront and, as an industry, need to involve patients from the very beginning and through consistent touchpoints throughout the trial.
The benefits of this practice increases enrollment, enables better retention, and creates a consistently thoughtful environment for the patient and their journey.
How Life Science Companies Leverage ScienceMedia's SMi Trial to Achieve Greater Compliance
Janssen Uses SMi Trial to Propel Sophisticated Site-Based Training
As discussed by Michael McKay during the panel, the team at Janssen was mission-aligned with ScienceMedia because Janssen needed a more sophisticated education and training program for their global studies to ensure sites were set to efficiently run their clinical trials. More than just the study startup, they too needed training on recruitment and enrollment for their staff to cover a holistic, ongoing training program through each phase of the trial. Janssen's rationale was to have a robust and professional training program that matched the investment of the studies they were operating.
Though Janssen recently adopted the use of ScienceMedia's SMi Trial, those using the modules have reported favorable experiences, continued use of the training modules, and significantly fewer reported deviations.
An added benefit of holding an efficient and effective training program for Janssen is it built the case to be a 'sponsor of choice' because they communicated to all of their stakeholders that they hold rigorous standards of training to deliver high-quality clinical trial experiences for those operating and enrolled. They wanted to do better for all those they served and continue to serve.
Record-Breaking SMi Trial Use at a Global Pharma Highlights Need for Enhanced Training
There are a few more examples, though blinded, that come to mind that highlight the need for enhanced training. A prominent, well-known global top five pharmaceutical company running a 400+ site Phase III trial operating in over 36 countries chose SMi Trial to yield strong site engagement by providing easy access to a central repository of vendor and study training materials. SMi Trial's platform assigned materials by country and by roles while providing traceable regulatory compliance.
There are a few more examples, though blinded, that come to mind that highlight the need for enhanced training. A prominent, well-known global top five pharmaceutical company running a 400+ site Phase III trial operating in over 36 countries chose SMi Trial to yield strong site engagement by providing easy access to a central repository of vendor and study training materials. SMi Trial's platform assigned materials by country and by roles while providing traceable regulatory compliance.
Below is an image from this study that supports the high usage and engagement from sites utilizing the SMi Trial approach. One piece of feedback that resonates particularly with ScienceMedia is a comment echoed by many site personnel, and that is: "I really enjoy the video presentations over PowerPoint," and this is because the team at ScienceMedia has seen the proven value of offering short videos to enable the site personnel to quickly attain useful information and go back to the videos over and over to recall and remain compliant.
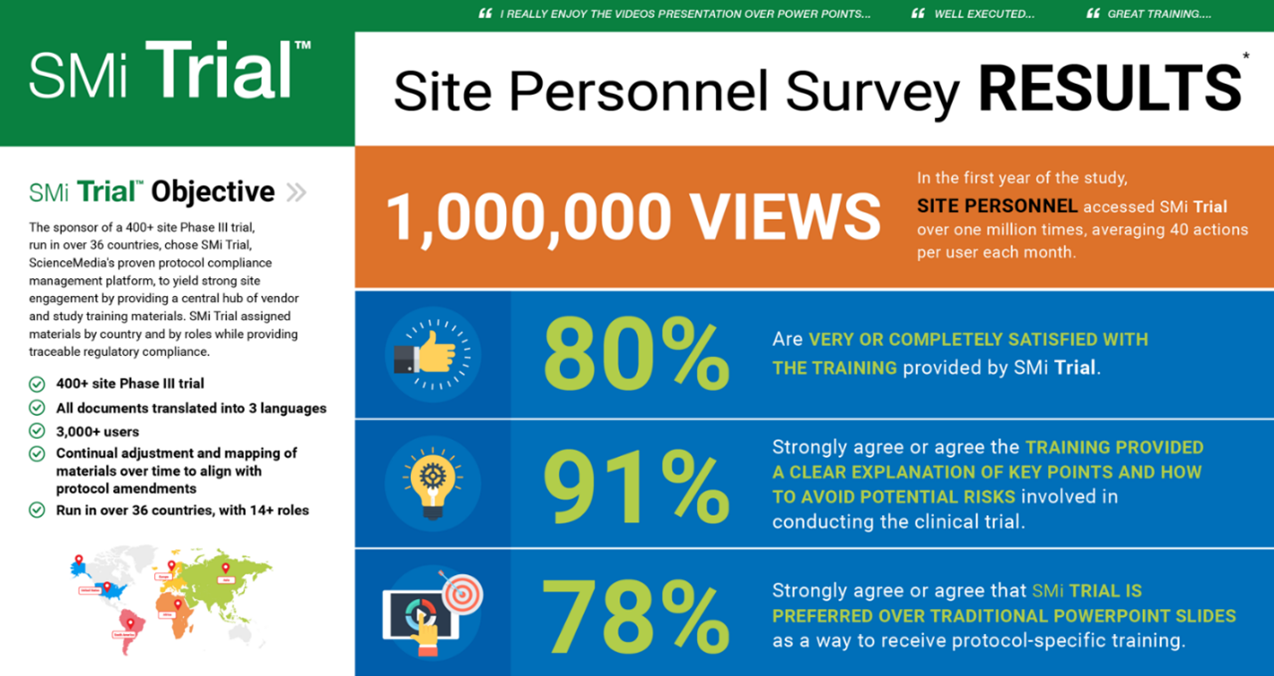
©ScienceMedia 2022. SMi Trial usage results.
SMi Trial Enables a Large, Global Pharma to Scale and Ensure Quality of Clinical Data
In this particular example, a large, global pharmaceutical company identified one of its investigational CNS drug programs as 'the future of the company.' But the program's multi-arm, Phase II trial was jeopardized by enrollment errors and protocol deviations despite the deployment of hands-on site training. The sponsor tasked their Clinical Research Organization (CRO) to help mitigate those risks during their global Phase III study. In order to scale the trial globally and ensure the quality of the clinical data, the CRO recommended and implemented SMi Trial.
SMi Trial was implemented to complete their study six months sooner, streamline enrollment, reduce subject costs, prevent protocol deviations, standardize compliance across 142 global sites and 40 Clinical Research Associates (CRAs), and ensure clinical data quality.
The result of this partnership includes:
FDA and EMA approvals ahead of schedule
Trial completion six months earlier
Deviation rates in the top 10% of all studies conducted in the CRO's history
$100,000s saved in the downstream monitoring and additional time-to-market opportunity costs
Significantly lower placebo rates. A key challenge for this client was high placebo rates required hands-on site training that would be impossible to implement globally
No adverse findings in audits of training, protocol compliance, and patient protection
No regulatory issues after multiple site and sponsor inspections
The value of SMi Trial to this client is further indicated in the graphic below.
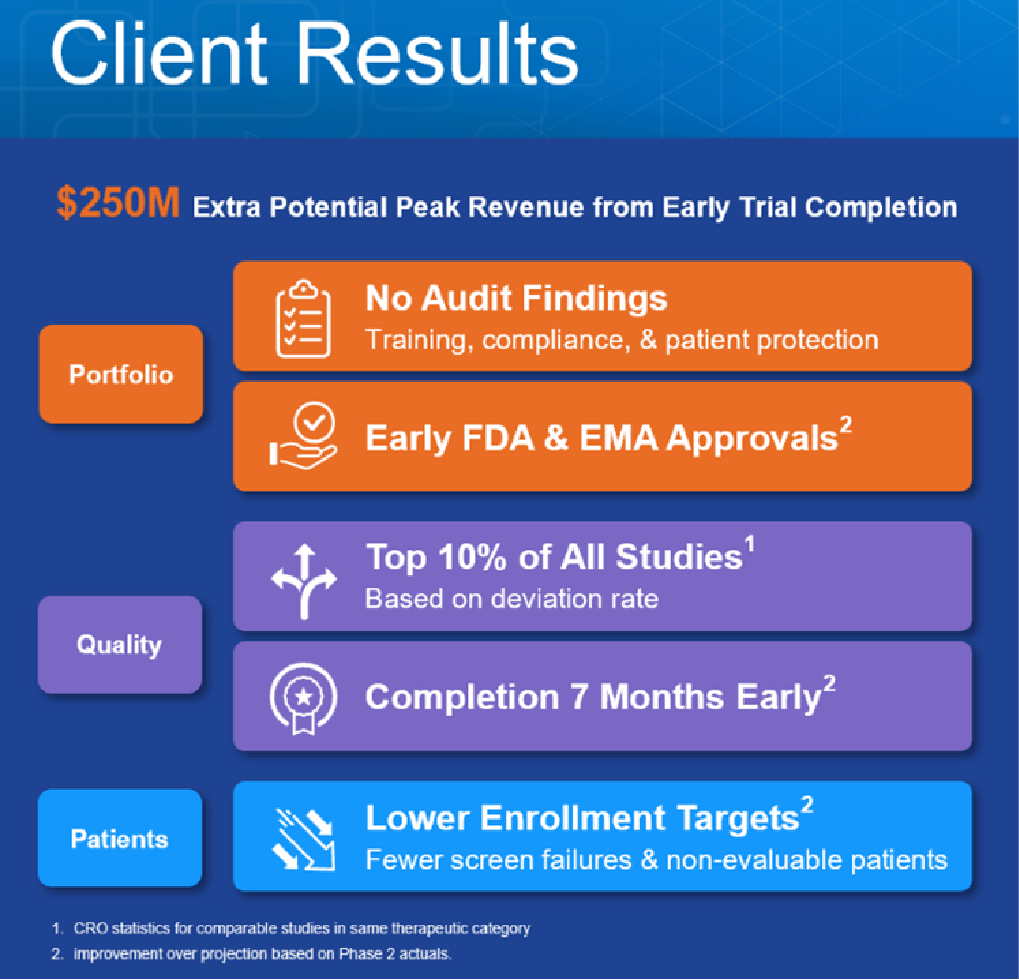 ©ScienceMedia 2021. SMi Trial usage results.
©ScienceMedia 2021. SMi Trial usage results.
Concluding Remarks
Site-based trial staff have enormous details to manage throughout the lifecycle of a clinical trial to remain compliant, not to mention typically supporting multiple trials from different sponsors at the same time, and the risks of deviations and errors are just too high without adequate training. SMi Trial, in particular, helps sponsors and CROs transition to online clinical trial management by facilitating global communication and monitoring of sites, circulating protocol training and study updates, and deploying comprehension assessments to identify the high-risk areas of trials without requiring on-site visits.
Even some of the best conducting clinical trials experience issues. Fundamentally, a well-functioning trial starts at the very beginning with proper planning, proper training, excellent identification of risks and issues, and continuous monitoring of those features throughout the lifecycle of the trial.
But we are also in an exciting time. As our industry rapidly evolves, it's time to make a change as traditional learning approaches need to meet the increasing demand for clinical competence. It's critical that sponsors provide compelling training solutions that meet this need, along with innovative delivery options for a hybrid workforce.
About CORE East
CORE East, the Clinical Operations Retreat for Executives, was launched in 2004 and is an invitation-only meeting that brings together an exclusive group of senior leaders in clinical development to discuss and debate the most pressing issues around the business of product development and building enduring companies in this space. Following the success of CORE East, CORE West was launched in 2021 with the same mission.
Want to learn more about CORE? Contact Maria Coakley, Events Manager.



 ©ScienceMedia 2021. SMi Trial mobile enablement.
©ScienceMedia 2021. SMi Trial mobile enablement.

 ©ScienceMedia 2021. SMi Trial usage results.
©ScienceMedia 2021. SMi Trial usage results.












